Biodistribution and dosimetry of [Lu-177]Lu‐SibuDAB in patients with metastatic castration‐resistant prostate cancer
Philipp Ritt, René Fernández, Cristian Soza-Ried, Heinz Nicolai, Horacio Amaral, Korbinian Krieger, Ana Katrina Mapanao, Amanda Rotger, Konstantin Zhernosekov, Roger Schibli, Cristina Müller & Vasko Kramer
Abstract
Background
Several prostate-specific membrane antigen (PSMA) radiopharmaceuticals have been used for the treatment of metastatic, castration-resistant prostate cancer (mCRPC). In an attempt to improve the tumour accumulation, new PSMA ligands were developed with an albumin-binding entity to enhance the blood circulation and, hence, tumour accumulation. In preclinical studies, [177Lu]Lu-SibuDAB, a radiopharmaceutical with moderate albumin-binding properties, outperformed [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T. The aim of this study was to evaluate the dosimetry of [177Lu]Lu-SibuDAB in patients diagnosed mCRPC.
Methods
Seventeen patients (median age 72 years, range 63‒83) diagnosed with progressive disease of mCRPC were included in this prospective study after exhausting all available treatment options. They were injected with 5.3 ± 0.5 GBq (mean ± standard deviation) [177Lu]Lu-SibuDAB as a first treatment cycle. Sixteen of these patients underwent sequential whole-body SPECT/CT and activity determination in venous blood samples for dosimetry purposes. Absorbed doses to the salivary glands, liver, spleen, kidneys, and red marrow as well as selected tumour lesions were calculated in OLINDA/EXM™ and compared to published values for previously established PSMA radiopharmaceuticals.
Results
Absorbed dose coefficients (ADC) to tumours (9.9 ± 5.4 Gy/GBq) were about 2-fold higher than those reported for clinically approved PSMA radiopharmaceuticals. ADC to salivary glands, liver, spleen, kidneys and red marrow were higher (0.5 ± 0.2, 0.2 ± 0.05, 0.2 ± 0.1, 1.8 ± 0.6, 0.1 ± 0.04 Gy/GBq, respectively) than for [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T, but lower than for [177Lu]Lu-PSMA-ALB-56, a previously investigated long-circulating PSMA radiopharmaceutical. The tumour-to-kidneys, tumour-to-red marrow, tumour-to-salivary glands ADC ratio were 6.6, 102, 33.1. These ratios were comparable to those of [177Lu]Lu-PSMA-617 and [177Lu]Lu-PSMA-I&T for kidneys and red-marrow, but higher for salivary glands.
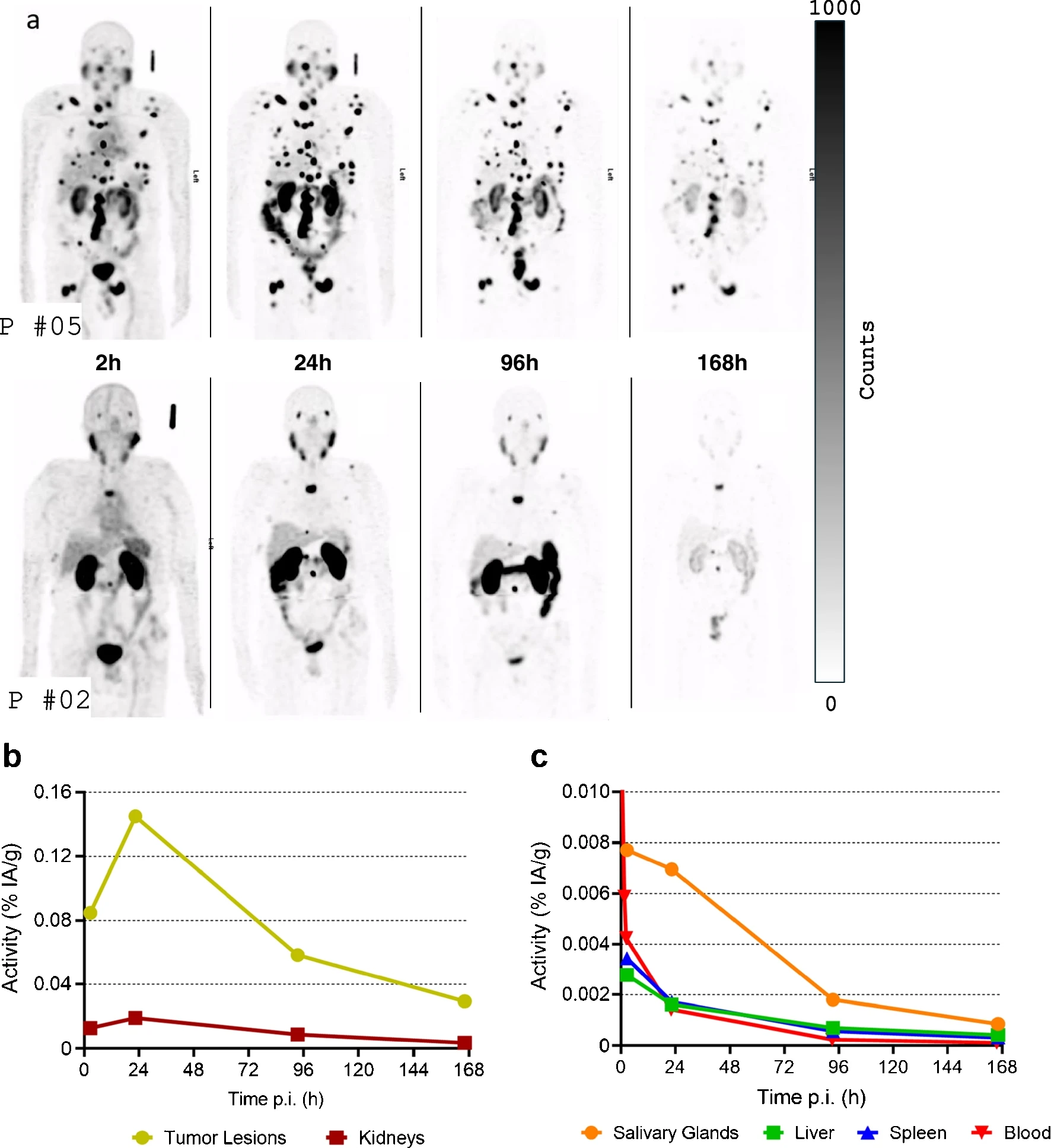 a) Maximum-intensity projections of SPECT images of [177Lu]Lu-SibuDAB patient #5 (medium total tumour uptake) and patient #2 (low total tumour uptake), windowing at all timepoints was adjusted so that bottom (white) is 0 counts and top (black) is 1000 counts; b) TACs for tumours and kidneys, expressed as percent injected activity per gram (% IA/g) for patient #5; c) TACs for salivary glands, liver, spleen and red marrow (blood) (% IA/g) for patient #5. For better visualization, the initial peak of blood TAC is not shown. P = patient, p.i. = post injection; TAC = Time-activity curve
a) Maximum-intensity projections of SPECT images of [177Lu]Lu-SibuDAB patient #5 (medium total tumour uptake) and patient #2 (low total tumour uptake), windowing at all timepoints was adjusted so that bottom (white) is 0 counts and top (black) is 1000 counts; b) TACs for tumours and kidneys, expressed as percent injected activity per gram (% IA/g) for patient #5; c) TACs for salivary glands, liver, spleen and red marrow (blood) (% IA/g) for patient #5. For better visualization, the initial peak of blood TAC is not shown. P = patient, p.i. = post injection; TAC = Time-activity curve
Conclusion
[177Lu]Lu-SibuDAB showed a prolonged blood circulation time and, hence, a significantly increased absorbed tumour dose, while tumour-to-organ ADC ratios were similar to conventional PSMA radiopharmaceuticals. Further clinical investigations to evaluate the efficacy and safety of [177Lu]Lu-SibuDAB are, thus, warranted.
Additional information
This article is licensed under a Creative Commons Attribution 4.0