Hermes Medical Solutions Receives 510(k) FDA Clearance for its Hermia NM Processing (Hybrid Viewer)
Hermes Medical Solutions (HMS), global market leader in molecular imaging and dosimetry software solutions receives 510(k) FDA Clearance for its Hybrid Viewer 7.0. The product has previously been CE-marked and holds a medical device license from Health Canada.
“We are proud to announce that we have received FDA 510(k) clearance for our new version of Hermia NM Processing. It includes the latest features used in molecular imaging and simplifies workflows significantly. This is a new milestone in NM Processing and emphasizes the advantage of separating procurement of cameras from workstations. With us, you can have the freedom to choose the best of both worlds as Hermes Medical Solutions is the only vendor neutral provider of molecular imaging software that fully supports all camera brands.” Tom Francke, CEO of Hermes Medical Solutions.
Hermia offers the most comprehensive Nuclear Medicine processing toolkit on the market. Fully validated applications, for all clinical specialties and associated image data types, follow intuitive steps to facilitate easy adoption within the desired clinical workflow. Below you can read what has been added to the newly FDA-cleared version.
New and improved features
- Various improvements and fixes to maintain compatibility with latest camera models from all vendors
- Alternative method of screen-captures to meet new PII requirements
- Improved support for reading and writing DICOM SEG files for PT studies
 Added functionality for Motion Correction with dual isotope studies
Added functionality for Motion Correction with dual isotope studies- Improvement for faster multiple region edits
- Automatic generation of Pseudo planar lung images from a tomographic acquisition study
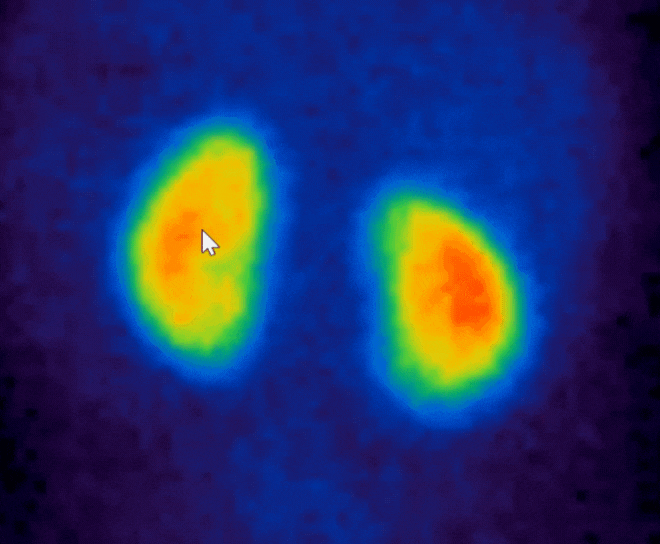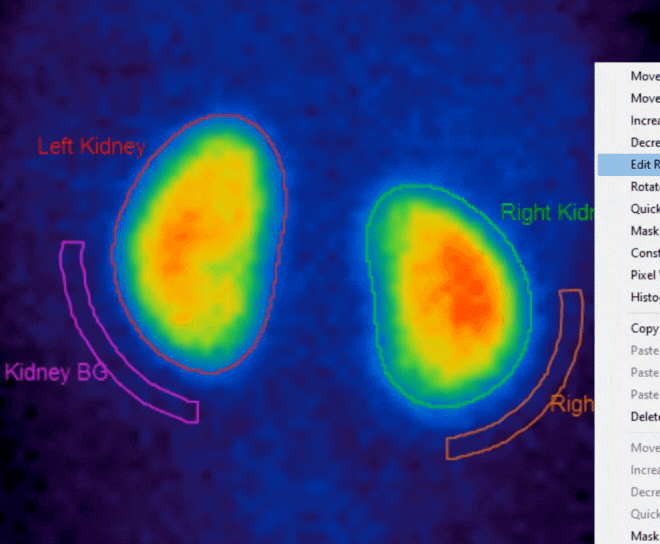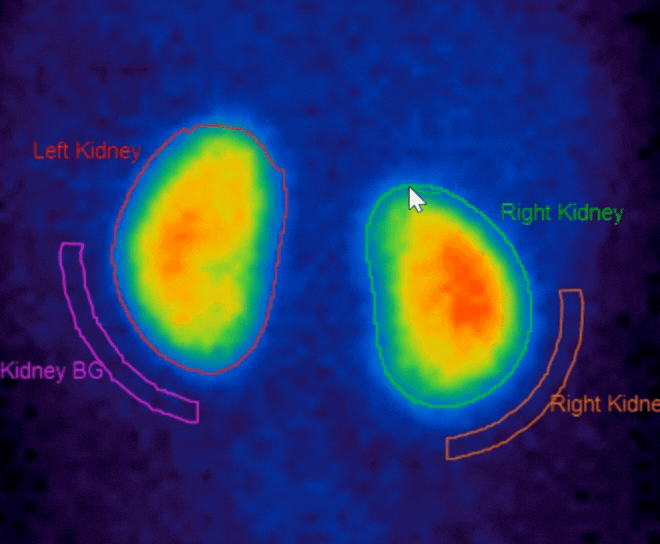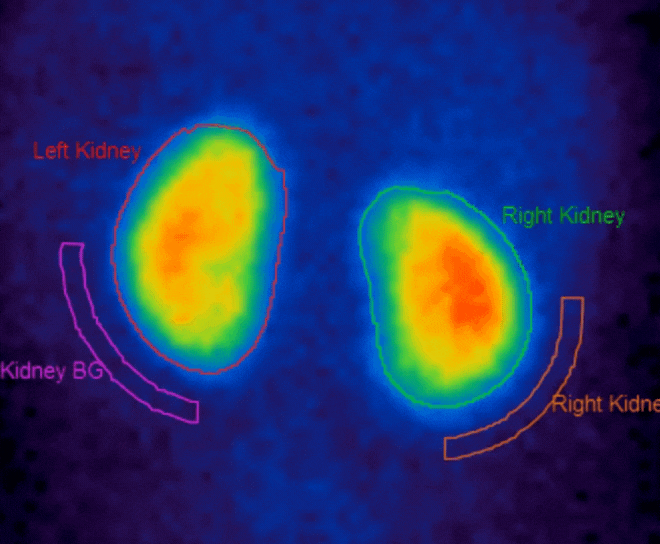
- DMSA n ow supports automatic region creation, motion correction for dynamic studies, and support for SPECT studies
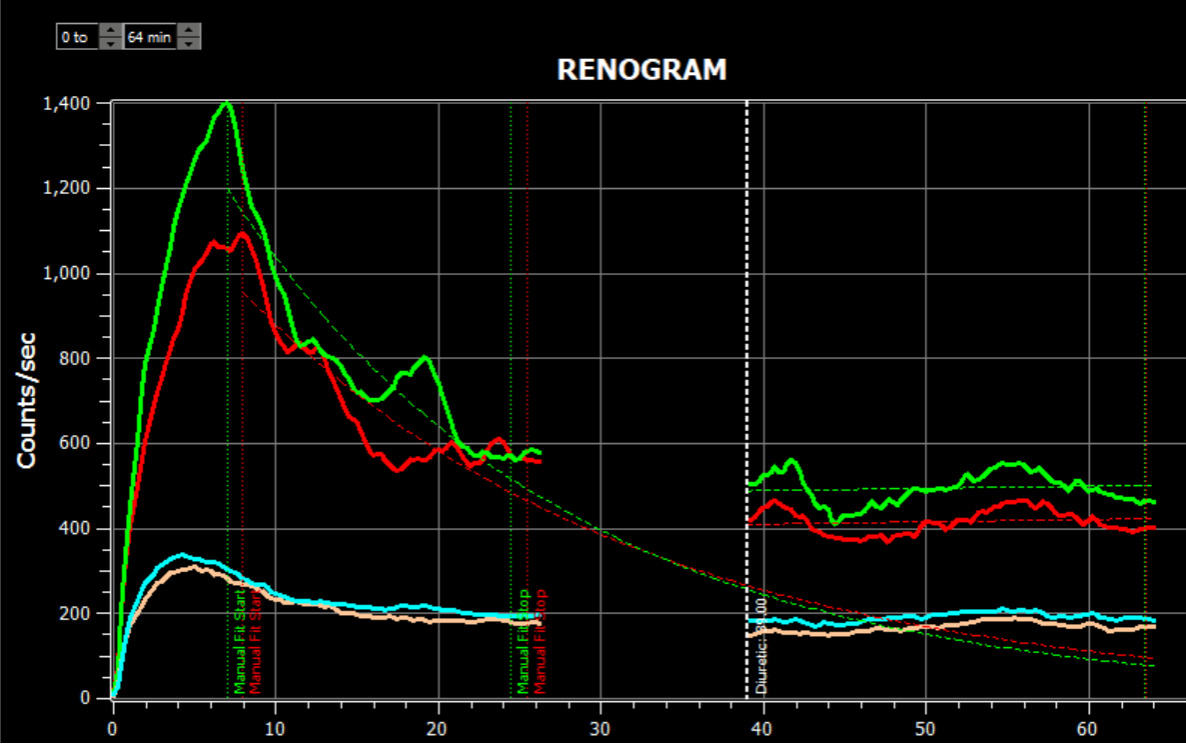
- Renogram
- Geometric mean analysis for post micturition dynamics
- Improved Combine tab with separate markers for each portion of the study
- New option to always skip last frame for result calculations
- Gastric Emptying
- Option to create an image layout for planar studies
- New result option based on Nottingham University Hospital calculations
- Organ Dosimetry
- Option to read regions drawn in Multimodality Viewer – Affinity
- Ability to delete individual ROIs which are part of VOIs
- Neurology and Cardiology studies rotated during reconstruction with Hybrid Recon keep their orientation on fusion with their CT
- Calculation of Efficiency factor added to Quality Control tools
- Gall Bladder: Additional markers and I-123 decay correction
- Salivary: Uptake and Relative Uptake Ratios now calculated on first dynamic of dual phase study
- Liver Remnant: Extra masking volumes added, and masking problems fixed
- Thyroid: Option to display thyroid image with and without ROIs and marker points
About Hermes Medical Solutions
Hermes Medical Solutions continuously innovates to enable faster and more personalized diagnosis and therapies in molecular imaging. With Hermia, we empower healthcare professionals with state-of-the-art software for all clinical scenarios into ONE vendor-neutral platform. Powerful tools enable clinicians to simplify their workflow and keep pace with the fast development of scanners, tracers and procedures in nuclear medicine. The result is improved quality in patient management and decision support for thousands of healthcare providers and their patients worldwide.
www.hermesmedical.com
Contact
Tom Francke
CEO, Assoc. Prof.
Hermes Medical Solutions AB (HQ)
Strandbergsgatan 16
112 51 Stockholm, Sweden
Tel: +46 70 166 1234
tom.francke@hermesmedical.com